Our Previous Work
Want an update on a HaPPeN trial?
Register your interest through the "contact us" link at the bottom of the page and the HaPPen team will add you to their mailing list and share the monthly HaPPeN newsletter updates. Alternatively follow the HaPPeN team on social media using the links below.

Protect neuro
Protect NEURO Study
Protect NEURO is a multi-centre international interventional cohort study evaluating the Neurocap (Polyganics BV, Netherlands) in the management of end neuromas.
Neuromas are painful nerve endings following trauma or amputation that result in severe neuropathic pain and functional impairment. There is no consensus on the optimum management of neuromas, however encapsulation has demonstrated benefit with capping devices, although compromised by late mechanical irritation.
The Neurocap TM is made from a polylactide of polycaprolactone bioresorbable polymer that softens over 12 weeks and is fully resorbed by 18 months. The Neurocap TM provides a temporary mechanical barrier to scar tethering of the nerve stump following surgical implantation after neuroma resection. The cap prevents mechanical irritation of the nerve, reducing fibrosis and pain. The chamber allows some unsupported axon outgrowth from the transected nerve stump which eventually involutes due to the absence of mechanical or neurotropic stimulation.
The success of the pilot STOP Neuro study led to roll out of this international commercially sponsored trial. Dominic Power was asked to be the Principle Investigator for the UK and the HaPPeN team is the world leading recruitment centre with 34 neurocap implantations in 22 of 73 recruited patients across 23 international centres of excellence in peripheral nerve surgery.
The trial results will be available by August 2020 and the 12 months follow-up data are to be presented at the American Society for Surgery of the Hand in September 2019.
Learn More
The Neurocap is a bioresorbable capping device for protecting nerve ends following neuroma excision. The trial has closed to recruitment but follow-up is ongoing. Final results will be available in 2020. Dominic Power is the Principle Investigator for UHB and the UK.
CaT-PINCH
CaT-PINCH: Carpal Tunnel Pinch grip responsiveness study
The CaT-PINCH study was developed with Professor Allan Wing in the SyMon Lab in the School of Psychology, Birmingham University. Dominic Power was the Principle Investigator at UHB for the HaPPeN team and the clinical component of the study was performed by Tom Pidgeon, as part of an Academic Foundation Training Post in the West Midlands Deanery.
The controls were recruited and tested at Birmingham University by Diar Karim, a doctoral research scientist in the SyMon Lab. Patients were recruited with suspected carpal tunnel syndrome and their disease severity was established using clinical scores, symptom severity scores and neurophysiological grading.
Patients were recruited to a one-stop carpal tunnel clinic at the Birmingham Hand Centre, providing clinical assessment, neurophysiology and treatment. Trial recruitment was from this clinic setting.
Patients were tested for grip responsiveness to perturbation using a validated test protocol. Following surgical decompression, patients were re-tested to establish whether the test protocol can correlate with disease severity and predict patients response to surgery. The study has closed to recruitment.
The preliminary results were presented at the British Orthopaedic association meeting in Liverpool in 2017. The final results of CaT-PINCH will be available in 2019.
Learn More
CaT-PINCH was a study undertaken by Tom Pidgeon as a part of his
Academic Foundation Training Fellowship with the HaPPeN team at UHB.
.png/:/rs=w:1240,h:620,cg:true,m/cr=w:1240,h:620)
MAP
MAP: Motor Assessment after Plexus injury
The Motor Assessment protocol after brachial Plexus injury study was conceived by Caroline Miller, the Chief Investigator and Physiotherapy Research lead at HaPPeN. Dominic Power advised on study design and is the PI.
The outcomes from brachial plexus reconstruction are ........
Learn More
MAP assess outcomes after BPI and defines whether motor strength and endurance correlates with functional improvement. To find out more contact Caroline Miller.
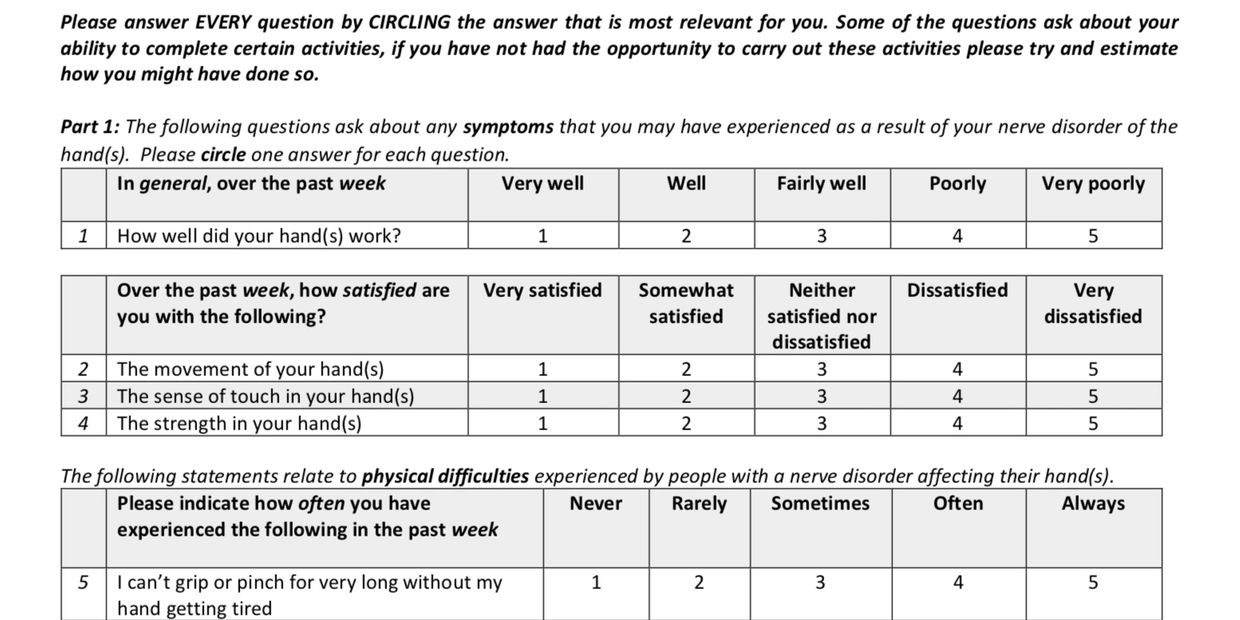
I-HaND: Impact of Hand Nerve Disorders
I-HaND PROM validation study
The I-HaND is a patient reported outcome measure (PRTOM) that was developed by Mark Ashwood and Professor Christina Jerosch Herold at the University of East Anglia. Following development clinical evaluation was undertaken at several UK test centres.
Caroline Miller, senior research Physiotherapist in the HaPPeN team collected datasets for validation through the West Midlands Brachial Plexus and Peripheral Nerve Injury Service.
The PROM is designed specifically with nerve dysfunction at the core and evaluates impact on patients lives. The PROM is being further evaluated by the HaPPeN team for use in digital nerve repair outcome assessment for use in a future multi-centre UK study of digital nerve repair.
Learn More
The I-HaND is a PROM designed to measure functional impairment and response to intervention for patients with conditions affecting hand nerve function. For more information on the I-HaND or other outcomes assessment after nerve injury, contact Caroline Miller or Suzanne Beale at HaPPeN.
EPIC
EPIC: Electronic Patient Information Communication
The EPIC pilot study was registered with C-Arms at UHB as a preliminary investigation to evaluate utility, acceptability and retention of digital information provision versus printed information sheets for patients following a flexor tendon repair within the hand at the Birmingham Hand Centre.
A DVD and electronic information including patient’s surgery and therapy following flexor tendon repair to include:
• Explanation of anatomy
• Description of surgery
• Description of Splint and therapy
• Precautions following surgery
• Advice on function and return to work
Background:
Patients have access to a wide range of digital media for information gathering. 98% of people living in the UK aged less than 40 years of age using the internet daily. A high incidence of traumatic hand injuries occur in 79-86% of the population less than 40 years of age and for this reason we proposed that digital information would be a useful tool in patient education and information provision.
The published literature reports that 50% of patients following flexor tendon rehabilitation want more information than is currently provided and that successful outcomes following surgery depend on adherence with post-operative therapy regimens. The design of the study was to develop and evaluate improved digital media for informing to patients about their flexor tendon injury and recovery.
Methods:
The audit was completed in two cycles with both groups containing 10 flexor tendon repairs. Patients were selected consecutively as they presented for surgery following a flexor tendon injury to the hand.
Group 1 were given standard patient information leaflet and verbal information at their first appointment. At the second appointment they were given a quiz on retaining information.
Group 2 were shown the video post operatively whilst in recovery and again at their first therapy appointment. At the second appointment they were given a questionnaire on the how useful they found the video.
Results:
- All patients across both groups reported they would be either likely or extremely likely to recommend our service to friends and family.
- All patients across both groups found the information relating totheir injury either useful or very useful.
- All patients felt confident in managing their injury.
- Only one patient felt they could have been given more information relating to theirinjury and this was from group 1, they reported wanting “more information on scar massage and time frames”.
- When asked: “Is there anything else our service can do to improve your experience?” one person from group 1 reported “yes, to some extent” but failed to expand further.
- Most patients felt that the video helped them understand their injury and were confident in managing their therapy.
- Patients perception of what is important to them is retained when information is given to them in both written and audio/visual format, such as purpose of splint, length of time needed to wear the splint and therapy frequency.
- Audio/visual delivery overall improved patients ability to retain more information.
- 100% of patients felt they understood what a flexor does and why we need to protect their repair.
Conclusion:
It is evident from this audit that patient information is important in patient’s understanding of their injury, surgery and rehabilitation. Future DVD’s have been produced on rehabilitation following injury/surgery mallet, central slip and extensor tendons.
Funding:
The EPIC study was funded by QEHB charities
Learn More
EPIC was a pilot prior to developing a portfolio of patient education digital resources. The project was supported by a pump-priming grant from UHB charities. The team are working on a number of other digital media patient education projects. Please get in touch if you would like to contribute, collaborate or support this activity.

SUBMIT
SUBMIT: Stability of Unicortical or Bicortical Metacarpal fracture Internal Fixation Trial
Metacarpal fractures are common and functionally displaced fractures may benefit from internal fixation with plates and screws. Drilling the bone and placing a screw through both cortices is the traditional method or orthopaedic fracture fixation but poses a small risk to the volar structures. Fracture complications including mal-union or non-union are rare and the study is designed to evaluate whether fixing with shorter unicortical screws is adequate for stability to achieve satisfactory bone union.
The study was developed in Birmingham with Katie Young, the first HaPPeN junior research fellow, Feiran Wu, an orthopaedic trainee in the West Midlands and Rajive Jose, a consultant in the Birmingham Hand Centre and Chief Investigator for the trial.
Pilot funding was received through a grant from the RCDM and the trial is adopted to the NIHR portfolio. Following the COVID-19 Pandemic, the trial was felt to be unlikely to achieve it's recruitment target and as such the decision to close the study was taken by the team.
Feiran Wu has now taken over from Col. Foster as the Chief Investigator and the data for the trial is being collated and analysed with an update expected later in 2023.
Learn More
SUBMIT is a multicentre study run through the HaPPeN team at the SRMRC, Institute of Translational Medicine, Birmingham, UK. Rajive Jose is the Chief Investigator. Contact us if you want your centre to be involved.

SCOPING
SCOPING: A Series of Clinical Observational Parameters Indicative of Nerve reGeneration
Suzanne Beale is the lead investigator for this rehabilitation study delivered through the HaPPeN team at UHB.
The aim of this study is to establish a standardised evidence-based protocol following median nerve repair, clinically evaluating measurements that may predict the need for early surgical intervention if the nerve is not progressing as predicted. The four main clinical assessment parameters used will be the Progressing Tinel, the Differential Tinel's sign, the Tender Muscle Sign and Thermographic Imaging.
SCOPING is an observational study that commenced recruitment in Q1 2018 at UHB. The study population is complete transection injury of the median nerve at the wrist or in the distal third of the forearm.
The primary outcome measure used will be the Mackinnon Modified British Medical Research council (MRC) sensory scale. The secondary outcome measures used will be the static and moving 2PD, Semmes Weinstein Monofilaments Test (SWMT), Medical Research Council (MRC) Motor scale, Pain scale, Cold Intolerance Scale, Hyperaesthesia and patient reported outcomes DASH, EQ5D and PEM.
Patients will be assessed at standardised intervals of 2 weeks, 6 weeks, 3 months, and additional research visits at 6 months, 9 months, 12 months and 18 months. We will then standardise the assessments required at each interval of treatment. All patients will be assessed and treated by the lead therapist.
Sample Size: This is a pilot sample size in an observational study. Working on 25-40 patients per year it is feasible to be able to recruit 12 patients to be assessed and reviewed over a period of 24 months allowing for over recruitment of 20%. The recruitment target is set at 0.5 patients per month.
Surgery: All patients will have undergone a repair of the median nerve within Zone VI (0-10 cms proximal to the volar wrist crease).
Inclusion Criteria:
- Adult patients (>18 years) sustaining acute hand injuries with median nerve division that can be directly apposed for surgical repair with no gap. Repair up to 10 cms proximal to the volar wrist crease.
Exclusion criteria:
- Patients with a history of local wound infection.
- History of prior injury to the median nerve.
- History of median nerve compression or cervical radiculopathy.
- History of conditions that may cause peripheral neuropathy.
- An inability to understand the examination instructions due to language barriers
- Patients not consenting to inclusion in the study.
- Pregnancy
- Prisoners
Data Collection:
1. Age, sex, occupation, hand dominance, co-morbidities
2. Date of injury
3. Date of surgery
4. Baseline sensory function
5. Consent for thermal imaging
6. Surgical findings
7. Operation performed – direct repair of the median nerve
Primary Outcome Measure:
1. Mackinnon Modified British MRC Sensory Scale.
The Mackinnon Modified British MRC sensory scale was chosen has the primary outcome measure as it is a recognised tool used in the literature and is less time consuming to use than other outcome measures.
Secondary Outcome Measures:
1. Medical Research Council (MRC) motor scale
2. Semmes-Weinstein Monofilament Test (SWMT)
3. Static and moving 2PD
4. Autonomic Grading scale- Likert scale
5. Pain Intensity Grading scale – Likert scale
6. Cold Intolerance Grading scale – Likert scale
7. Hyperaesthesia Grading scale – Likert scale
8. Disabilities of the Arm, Shoulder and Hand (DASH) 2
9. Patient Evaluation Measure (PEM) questionnaire
10. EQ5D
Grant funding:
This study has been funded by QEHB charities.
References:
- Wang Y, Sunitha M, Chung K (2013) “How to Measure Outcomes of Peripheral Nerve Surgery” Hand Clinics June 2013.
- Hudak P, Amadio PC, Bombardier C, and the Upper Extremity Collaborative Group. Development of an Upper Extremity Outcome Measure: The DASH (Disabilities of the Arm, Shoulder, and Hand). American Journal of Industrial Medicine 1996; 29:602-608.
Learn More
Following nerve repair, there is a significant lag before the clinical outcome is established due to the slow rate of axon regeneration. The SCOPING study is examining clinical tools that may predict failure and prompt early re-exploration and reconstruction.

COMBINE
COMBINE: Core Outcome Measures in Brachial plexus INjury Evaluation
Caroline Miller leads the COMBINE study which has established an international patient and clinician core outcome dataset for reporting outcomes after adult traumatic brachial plexus injury. The study is in the final phase and dissemination through presentations and publications is underway.
Learn More
COMBINE is a study to define a core outcome assessment dataset for measuring patients after brachial plexus injury and comparing different treatment strategies.
